Information Cascades Among Ciliated Protozoa
 |
 |
 |
|
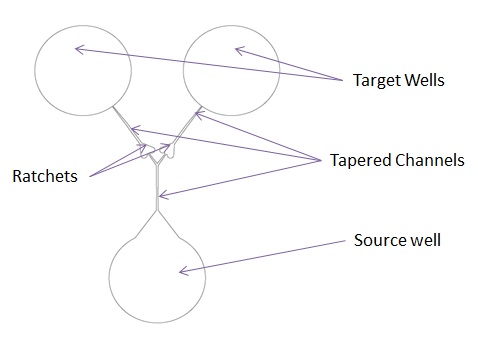
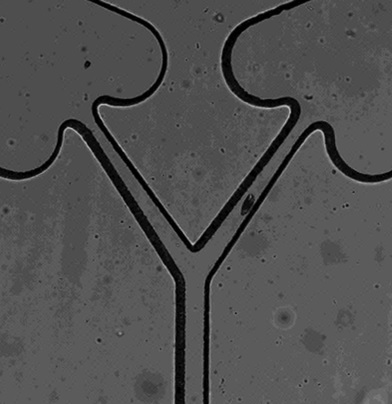
Device concept with a single source well splitting into two channels. Constrictions along channels help ensure one-direction migration. |
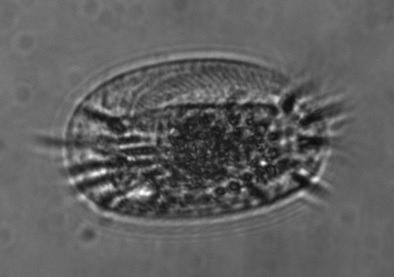
Protist crawling through a microfluidic device: shown is an individual of the species Euplotes vannus moving through a water-filled channel 55 Ám wide. |
Target organism used is Euplotes vannus, a common marine ciliate. |
The objective of this project is to test the hypothesis that protozoa leave durable chemical signals, like ants. We used a microfluidic device to force protozoa to move from a source well to one of two target wells single-file, and tracked the directions of sequential protozoa. Chemical trails may influence the movement of protozoa in natural environments, their ability to locate food, and their ability to recycle nutrients.
People Involved: Paige Orlofsky, Grant Bouchillon, Mike Shor
Protozoa Migration
Most microbial habitats in nature, industry, and in vivo have complex physical structures. In the environment, physical microstructures including intra-particle pores and cracks and inter-particle voids provide bacteria with refuge from predation. The limitations on protozoa migration by micro-structured channels has been investigated with microfluidic devices.
Mobility in Narrow Channels
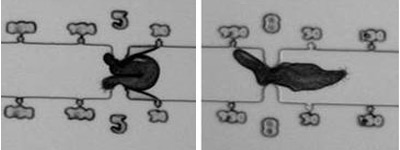 Keronopsis sp. through 35 um (left) & 20 um wide constrictions.
Keronopsis sp. through 35 um (left) & 20 um wide constrictions.
The ability of five species of ciliated estuarine protozoa to pass short or long constrictions was measured using microfluidic devices. The protozoa tested survive in PDMS microfluidic devices for a week or longer. During the size exclusion experiments, protozoa were observed to readily enter narrow channels and to pass constrictions smaller than their unconstrained cross-sectional area. (view a movie).
See Mobility of Protozoa through Narrow Channels, Applied & Environmental Microbiology 2005, 71:4628-4637.
Migration in Bent Channels
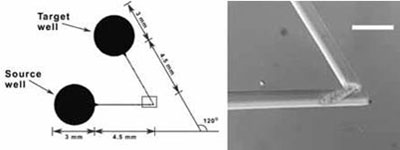 (left) A bent microchannel connects two wells. (right) Photomicrograph of an E. vannus individual traversing a 120° channel bend. Scale bar 100 um.
(left) A bent microchannel connects two wells. (right) Photomicrograph of an E. vannus individual traversing a 120° channel bend. Scale bar 100 um.
The ability of ciliated estuarine protozoa to pass channel bends of 60°, 90°, or 120° was measured using microfluidic devices. Findings include:
- Protozoa travel more slowly in constricted channels.
- Channel bends slow protozoa migration, with acute angles limiting migration the most.
- Movie files:
- Movie of several E vannus traveling in opposite directions at channel bend.
- Movie of several E vannus reticulating before a 60° channel bend.
See Protozoan Migration in Bent Microfluidic Channels, Applied & Environmental Microbiology 2008, 74:1945-1949.
Dissertation work of Wei Wang. In collaboration with David Kosson, Eugene LeBoeuf, Gary Taghon, and John Wikswo.