Microengineering the Bioflim Microhabitat for Treatment of Disease
Recently published articles: Deng,
Jinzi, et al. "Dynamic dosing assay relating real-time respiration
responses of Staphylococcus aureus biofilms to changing microchemical
conditions." Analytical chemistry 85.11 (2013): 5411-5419.
 |
Biofilms are the dominant growth form of bacteria in most
natural environments, as well as in many industrial and in vivo
settings. In the medical context, biofil m-associated bacteria are
often associated with chronic infection and poor clinical outcomes.
Unlike homogeneous liquid suspensions, biofilm-associated bacteria
exhibit dynamic responses that are dissociated from their immediate
surroundings, that vary with position, and that evolve over time.
Existing antimicRerobial screening techniques either do not maintain
the essential biofilm morphology and/or do not capture the dynamic
nature of their responses. Typically, antimicrobials are tested
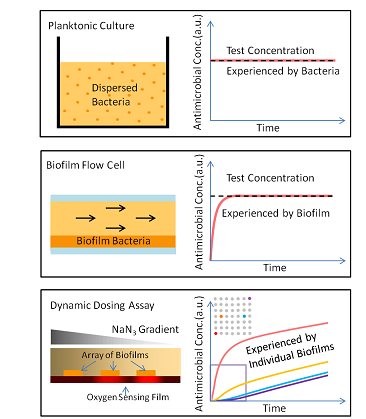
using either planktonic bacteria suspended in well-mixed solution or a biofilm, such as in a flow cell of the CDC
bioreactor. In either case, antimicrobial concentration is
constant in vitro despite the fact that dosing rate is an essential
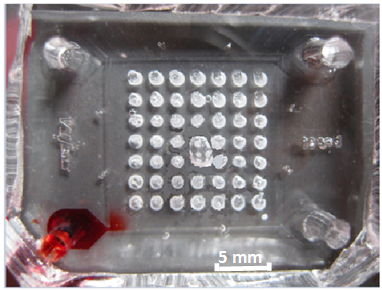
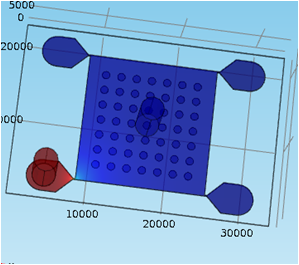
factor in vivo. We developed the first assay for testing responses of biofilms to antimicrobial dosing rate. In the system, dozens of identical biofilms can be screened for real-time responses to dynamically changing antimicrobial concentrations1 (Fig. 1,2,3). This work resulted in a comprehensive publication in Analytical Chemistry in 2013 that describes the device design and operation, including biofilm patterning, gradient control, and real-time analysis using a spatially continuous oxygen sensing film. Biofilm oxygen utilization rates could be modeled based on fluorescent intensity change of oxygen sensing film (Fig. 4) underneath the biofilm over time.
|
||||||
| Figure 1. Methods to screen antimicrobials include (A) well-mixed planktonic culture, and (B) steady-biofilms operated at steady state. In contrast, (C) our dynamic dosing assay measures responses of an array of biofilms as a function of dosing rate of up to four antimicrobials. Here, a side view shows two biofilm “dots” responding to different antimicrobial concentrations diffusing from the left. Time-dependent respiration is determined by oxygen-quenched fluorescence of an immobilized reporting molecule. Graph shows simulated antimicrobial concentration vs. time at array positions shown in the inset. |
People Involved: Jinzi Deng
In collaboration with Adit Dhummakupt, Philip Samson, John Wikswo at VIIBRE (Vanderbilt University)
Funding: UConn Faculty Large Grant, UConn Summer Undergraduate Research Fellowship, NSF 0649883, the USDA through AFRI 2011-0373, the US Defense Threat Reduction Agency grant HDTRA-09-1-0013